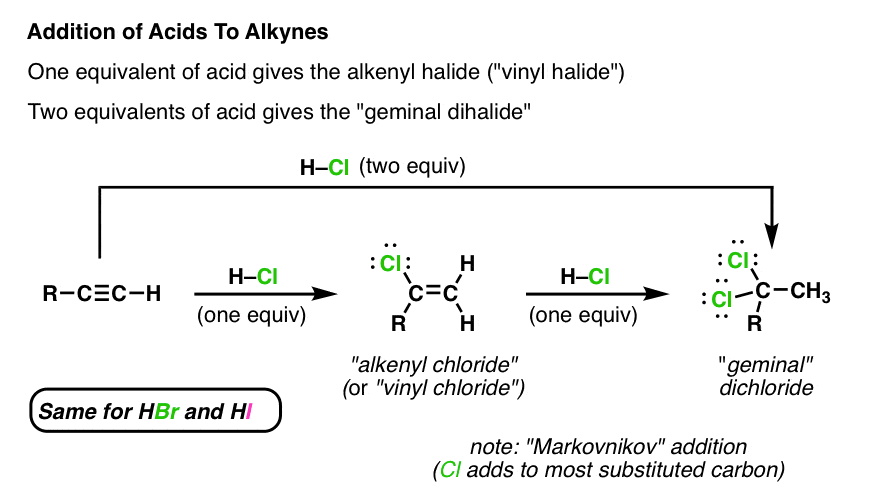Allylic carbocations like allylic radicals have a double bond next to the electron deficient carbon.
Vinyl vs allylic carbocation stability.
Allyl form a stable carbocation because of the electron delocalization.
Illustrates the resonance stabilization of allylic carbocation.
A vinyl carbocation has a positive charge on the same carbon as the double bond.
The allyl group allylic position is the next to a double bond cc c h h allylic carbon vinyl carbon allylic hydrogen sp2 hybridized vinyl hydrogen oh cl allyl alcohol allyl chloride 10 2.
We know that the rate limiting step of an s n 1 reaction is the first step formation of the this carbocation intermediate.
The vinyl cation is a carbocation with the positive charge on an alkene carbon.
Its empirical formula is c 2 h 3 more generally a vinylic cation is any disubstituted trivalent carbon where the carbon bearing the positive charge is part of a double bond and is sp hybridized in the chemical literature substituted vinylic cations are often referred to as vinyl cations and understood to.
Stability of carbocation intermediates.
In the allylic group if the allylic carbon atom carries a positive charge it forms an allylic carbocation.
The allyl cation is the simplest allylic carbocation.
Vinylic carbocations are unstable as they lack p character.
Difference between allyl and vinyl general molecular formula.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation.
The rate of this step and therefore the rate of the overall substitution reaction depends on the activation energy for the process in which the bond between the carbon and the leaving group breaks and a carbocation forms.
Allyl group gets attached to any other group of atoms through.
Allyl groups have three carbon atoms and five hydrogen atoms.
Do not confuse an allylic group with a vinyl group.
Allylic carbocations carbocation with a vinyl group as a substituent next to a double bond cc c 221 allyl carbocations are stabilized by.
The key difference between these two structural components is the number of carbon and hydrogen atoms.
As the allyl cation has only one substituent on the carbon bearing the positive charge it is primarily allylic carbocation.
The general molecular formula is rch 2 ch ch 2.
Due to the stability of the carbocation allyl compounds radially form intermediates during the reaction.
Therefore the stability order of carbocation can be written as.
Tertiary carbocation secondary carbocation primary carbocation.
This is very very unstable and ranks under a methyl carbocation in stability.
The allylic carbocation is stable due to delocalization of electrons on carbon atoms.
For example s n 1 reaction.
Allylic carbocations are able to share their burden of charge with a nearby group through resonance.